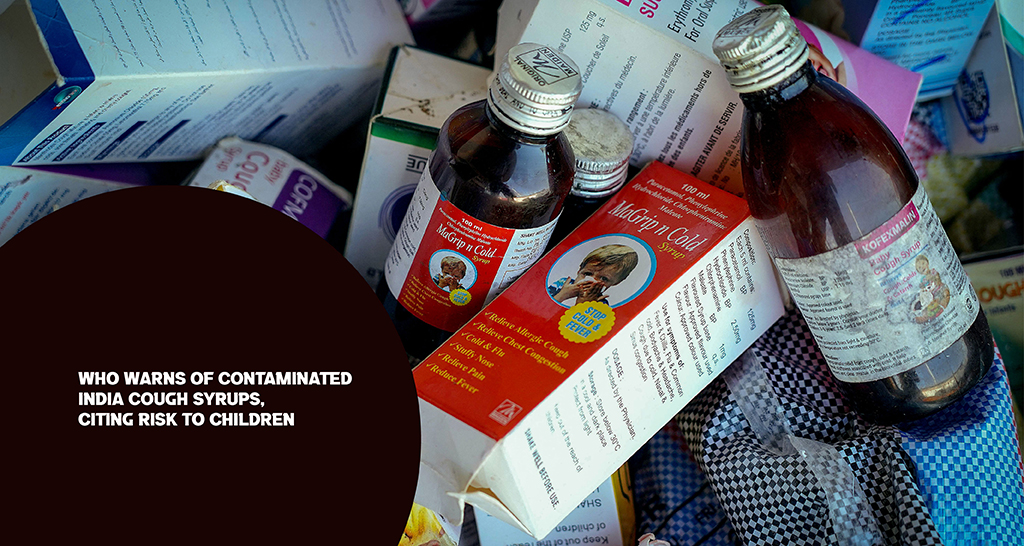
The World Health Organization (WHO) has raised a serious alarm after discovering cough syrups made in India that are dangerously contaminated. This has sparked new worries about the safety and quality of pharmaceuticals. The warning comes on the heels of reports of child fatalities in India linked to these harmful medicines, which contain toxic industrial chemicals.
According to officials from the World Health Organization, lab tests have shown that three brands of cough syrup Coldrif, Respifresh TR, and ReLife were found to have dangerously high levels of diethylene glycol (DEG). This chemical is typically used in antifreeze and industrial solvents. Even in tiny amounts, DEG can lead to serious health issues like kidney failure, neurological damage, and even death, particularly in children.
Health officials in India have confirmed that at least 17 children in Madhya Pradesh tragically lost their lives after consuming a contaminated syrup. In response, the government has put a stop to the sale of these products and initiated a nationwide investigation to uncover how these toxic batches made their way into the market. The manufacturing licenses of the companies involved have been suspended, and one factory owner has been arrested as part of the ongoing inquiry.
The WHO has issued a warning, urging countries to enhance their surveillance and testing of both imported and locally made cough syrups. They highlighted that these medications might have spread to other areas through informal or unregulated trade routes. The agency pointed out that the contaminated batches were intended for domestic use, but they also warned that the possibility of cross-border circulation can’t be completely dismissed.
In India, state governments are acting by recalling the affected products from pharmacies and hospitals. Health departments have also urged doctors to avoid prescribing cough syrups to infants and young children unless necessary. Meanwhile, the national drug regulator has mandated a thorough audit of pharmaceutical companies to pinpoint any shortcomings in testing and quality assurance.
This incident recalls similar heartbreaking events from recent years, where cough syrups produced in India were tied to the tragic deaths of numerous children in places like Gambia, Uzbekistan, and Cameroon. In those cases, diethylene glycol and ethylene glycol were found both of which are deadly if consumed. This troubling trend has led to increased international scrutiny of India’s pharmaceutical exports, which are a crucial supply source for many developing countries.
Public health experts are pointing out that this latest episode really shines a light on the ongoing issues with drug safety oversight. They emphasize that many smaller manufacturers depend on chemical suppliers who lack proper certification, and the regulatory testing process is often inconsistent. To avoid future disasters, they believe we need to strengthen enforcement, ensure transparent monitoring of the supply chain, and establish independent testing laboratories.
The WHO has announced that its actively collaborating with Indian authorities to assess the level of contamination and to make sure that no more batches of the harmful syrups are still out there. Additionally, the agency is thinking about issuing a formal global medical product alert to inform other countries.
The warning highlights the pressing need for rigorous pharmaceutical quality control and better international collaboration. As one health official pointed out, “These tragedies are not just accidents; they are preventable failures. Every child who loses their life to toxic medicine is a stark reminder that we need to be more vigilant on a global scale.”